The source of Earth’s water is an enduring mystery that extends to exoplanets and the notion of habitability. In broad terms, Earth’s water was either part of the planet from the beginning of its formation in the solar nebula or delivered later, maybe by asteroids and comets.
New research suggests that the Sun’s relentless solar wind could’ve played a role.
Scientists have worked hard to understand how Earth has so much life-giving water. There’s lots of research supporting the asteroid/comet delivery scenario. There’s also evidence that it accumulated water as it grew. During its accretion phase, it may have absorbed water-rich planetesimals.
To try to understand how Earth’s water fits into the history of the planet and the Solar System, researchers examine the isotope ratio on Earth and in meteorites. The isotopic composition of Earth’s water is most similar to primitive meteorites. On the other hand, it’s different from that of comets and nebular gas.
This implies that Earth’s water came from the same cosmochemical reservoir that is also the source of primitive meteorites.
It’s a complicated issue. Maybe Earth’s water has multiple sources. Maybe some of it was created in space long after Earth and the rest of the Solar System formed, and then delivered to Earth.
New research in The Astrophysical Journal explores how water can be created by the solar wind as it strikes surfaces holding oxygen-containing minerals. It’s titled “Stellar Wind Contribution to the Origin of Water on the Surface of Oxygen-containing Minerals.” The lead author is Svatolpuk Civiš from the J. Heyrovský Institute of Physical Chemistry at the Czech Academy of Sciences in Prague.
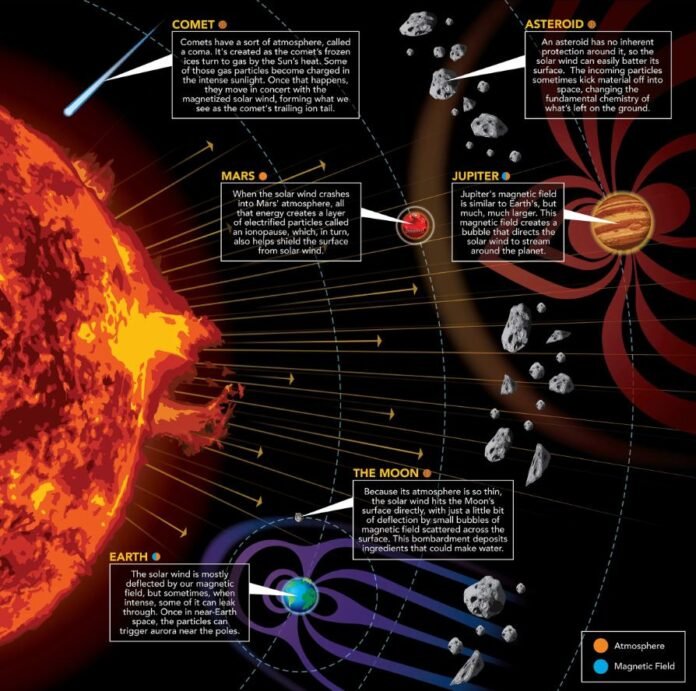
The solar wind is a steady stream of charged particles—mostly protons and electrons—that come from the Sun. H+ ions, which are simply protons, are the most abundant particles in the solar wind. They make a big contribution to the solar wind’s properties. Could the wind trigger the creation of water molecules?
The researchers performed laboratory experiments to find out. They tested 14 oxygen-containing minerals. “To investigate the process of water formation on the surface of oxidic materials and water abundances, we used the technique of surface bombardment with hydrogen or deuterium atoms and ions,” the authors write in their paper.

The experiments had two phases: the first tested whether the minerals would produce water when exposed to the solar wind, and the second tested their adsorption capacity. Separate from absorption, adsorption is the adhesion of a sample to a surface.
The team produced water and then measured it using two methods: a microwave (MW) discharge experiment and sputter gun irradiation. They tested the results with a type of spectrometry analysis called Fourier-transform infrared spectrometry (FTIR) and temperature-programmed desorption (TPD) analysis.
“Both these experiments include a mineral sample bombarded by hydrogen/deuterium ions, which, among other possibilities, react with surface oxygens in the mineral lattice and form water molecules,” the authors write.

The oxide material samples were not only exposed to the strong current of H, H+ and molecular hydrogen that mimic the solar wind. They were also exposed to intense visible and UV radiation generated in the hydrogen discharge.
“The stellar wind irradiation of rocky oxygen-containing minerals results in a reaction between H+ ions and silicate minerals to produce water and OH, which could explain the presence of water in the regoliths of airless worlds such as the Moon, as well as the water abundances in asteroids,” the authors write.
Previous research has established that a chemical reaction occurs between hydrogen ions and silicate minerals when rocky materials are exposed to solar wind irradiation. Some researchers have observed the formation of OH (hydroxide) and water, while others have only found OH. This research goes deeper by testing the rocky materials for water adsorption.
The researchers tested the samples’ water adsorption capacity. Then, they calculated how much material would need to reach Earth to account for the amount of water on contemporary Earth.
“Besides material acquired by the Earth during accretion, the solar wind origin of water and its delivery to Earth could have gone on even during post-accretional bombardment,” the authors write. Here, they’re referring to the hypothetical Late Heavy Bombardment.
Previous research shows that ” asteroid and comet impacts during the classical Late Heavy Bombardment would bring in about ?1020 kg of material,” the authors write. “If that material’s surface was fully saturated with adsorbed water as composed of one of our minerals, our calculations suggest that at least one ocean equivalent of water could have been brought in.”

There’s not much doubt about the results of these tests and the ability of the solar wind to create water.
“The results of the experiments summarized in this work, focused on surface bombardment with hydrogen atoms, clearly confirm the theory of the interaction of excited hydrogen or deuterium Rydberg atoms and ions with the surface oxygens of oxide minerals,” the authors explain. “Our experiments attempt to explain the origin of water in the areas of oxygen-containing solid material (e.g., dust, meteoroids, asteroids, comets) exposed to a stream of charged particles close to a parent star.”
Earth’s atmosphere and magnetosphere shield it from the solar wind, so there’s no way the wind could’ve created water right on Earth’s surface. However, as the study shows, the wind can create water on the surface of other bodies like asteroids, and the water can be adsorbed and held firm, then delivered to Earth via impacts.
“This scenario is also applicable to the origin of water on Earth,” the authors write. “Due to this effect, a water molecule can be adsorbed on the surface of oxygen-containing particles and then transported over long distances and times,” the researchers write.
This study won’t be the end of the ongoing effort to account for Earth’s water. In a fascinating roundabout way, this research brings us back to asteroids and meteorites delivering Earth’s water. If it can happen here, it can happen on exoplanets elsewhere in the galaxy.